Drug Search API & Plugin
Painless drug searches at your fingertips
Easily find, filter, and track drugs by ingredient, brand names, routes, and more.

Drug Search API & Plugin
Easily find, filter, and track drugs by ingredient, brand names, routes, and more.
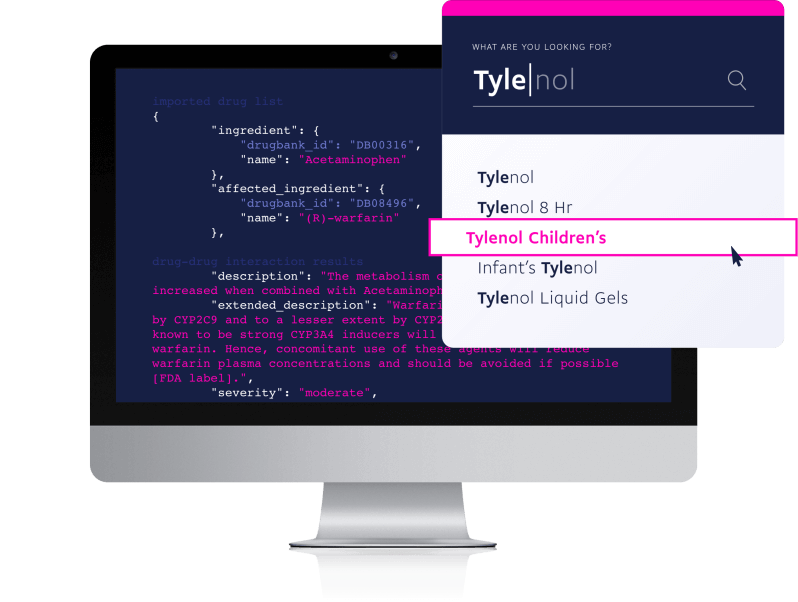
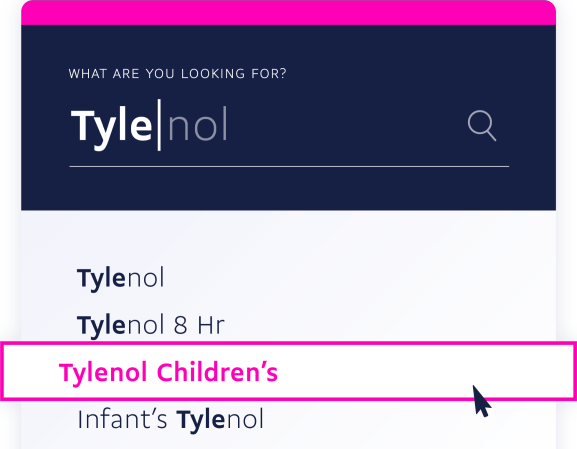
With autocomplete and fuzzy search enabled you can quickly and easily find the exact level of detail you need for any drug or medication.
Search by ingredient, prescribable, brand, or generic name.
This included feature is one of our most powerful search functions and allows you to:
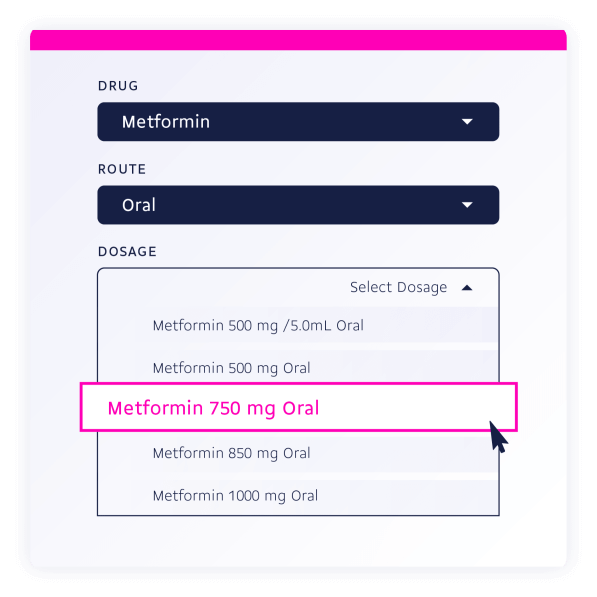
GET https://api.drugbank.com/v1/us/product_concepts/DBPC0009720/strengths[
{
"name": "Metformin 500 mg /5.0mL Oral",
"display_name": null,
"drugbank_pcid": "DBPC0232440",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "500 mg /5.0mL",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2004-01-04",
"regions": {
"us": true,
"canada": false,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Metformin",
"drug": {
"name": "Metformin",
"drugbank_id": "DB00331"
},
"strength": {
"amount": "500.0",
"per": "5.0",
"units": "mg/mL"
}
}
]
},
{
"name": "Metformin 500 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0009722",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "500 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1993-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Metformin",
"drug": {
"name": "Metformin",
"drugbank_id": "DB00331"
},
"strength": {
"amount": "500.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Metformin 750 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0009754",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "750 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2005-04-01",
"regions": {
"us": true,
"canada": false,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Metformin",
"drug": {
"name": "Metformin",
"drugbank_id": "DB00331"
},
"strength": {
"amount": "750.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Metformin 850 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0017628",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "850 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "1995-12-31",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Metformin",
"drug": {
"name": "Metformin",
"drugbank_id": "DB00331"
},
"strength": {
"amount": "850.0",
"per": "1.0",
"units": "mg"
}
}
]
},
{
"name": "Metformin 1000 mg Oral",
"display_name": null,
"drugbank_pcid": "DBPC0017612",
"brand": null,
"level": 3,
"route": "Oral",
"form": null,
"strengths": "1000 mg",
"standing": "active",
"standing_updated_at": "2018-09-12",
"standing_active_since": "2002-01-25",
"regions": {
"us": true,
"canada": true,
"eu": false
},
"rxnorm_concepts": [],
"ingredients": [
{
"name": "Metformin",
"drug": {
"name": "Metformin",
"drugbank_id": "DB00331"
},
"strength": {
"amount": "1000.0",
"per": "1.0",
"units": "mg"
}
}
]
}
]Included with our API, this powerful tool is ready to start making your life easier.
It's been beautifully designed with user-ease in mind and can take you from ideation to development in no time.
Contact our sales team to add
Drug Search to your software.